I’m assuming that someone actually has weighed the stuff. But my question is, How does flying in a plane create X tons of CO2? If CO2 has mass, then is new mass actually being created? How can new mass be created on a sealed planet? Or, is it simply changing mass from one thing to another?
How does burning 1 pound of jet fuel, create 6.97 pounds of CO2? https://impactful.ninja/the-carbon-foot ... -jet-fuel/
Does the earth have more mass than it did 100 years ago, since we’ve burnt so much fossil fuels?
How does a ton of CO2 work?
-
Josh
Re: How does a ton of CO2 work?
Take 1 molecule of natural gas, CH4, and combine it with 2 molecules of oxygen, O2RZehr wrote: ↑Mon Nov 20, 2023 12:23 pm I’m assuming that someone actually has weighed the stuff. But my question is, How does flying in a plane create X tons of CO2? If CO2 has mass, then is new mass actually being created? How can new mass be created on a sealed planet? Or, is it simply changing mass from one thing to another?
How does burning 1 pound of jet fuel, create 6.97 pounds of CO2? https://impactful.ninja/the-carbon-foot ... -jet-fuel/
Does the earth have more mass than it did 100 years ago, since we’ve burnt so much fossil fuels?
You get: 1 C, 4 H, and 4 O
That turns into 1 C and 2 O, or 2 CO2, and 2 H and 1 O, or 1 H2CO.
So CH4 + (2X) O2 -> (2X) CO2 + H2O
Most of the actual weight is in the oxygen. You can think of it as the oxygen in the atmosphere getting "converted" to carbon dioxide. A typical fuel ratio in a gasoline engine is 13 parts air to 1 part gasoline. Of course, the water that comes out is a significant part of the output weight too, but nobody currently worries about generating water.
It ends up being much ado about nothing since CO2 levels are quite stable and ocean algae populations vary as there is more CO2 to consume, and they turn it back into oxygen.
0 x
-
Ken
- Posts: 18626
- Joined: Thu Jun 13, 2019 12:02 am
- Location: Washington State
- Affiliation: former MCUSA
Re: How does a ton of CO2 work?
Yes, Josh is right.
In a chemical reaction, matter is neither created nor destroyed, it just changes form.
The increase in mass comes because in the combustion reaction, lighter hydrogen atoms are being stripped away from the jet fuel and are being replaced by heavier oxygen atoms. Which are coming from the atmosphere.
Petrochemicals like methane, gasoline, diesel, kerosene, coal, oil, etc. are hydrocarbon chains which means they are strands of carbon with hydrogen attached. The longer or bigger the hydrocarbon chain the more dense the liquid. Methane with 1 carbon is a gas, gasoline with 8 carbons is a liquid, motor oil and heavy greases have more carbons still, and bituminous coal, a solid, has 137 carbons.

When any hydrocarbon combusts it reacts with oxygen to form carbon dioxide and water. The simple reaction of methane is clean and just produces those two products and releases energy in the form of heat and light.
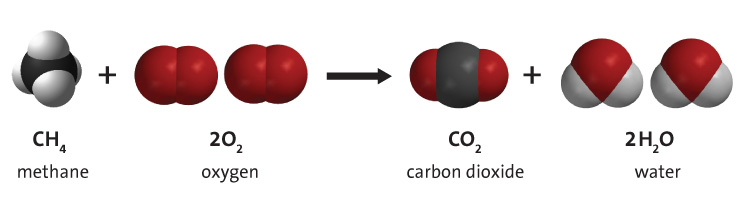
When larger hydrocarbons like gasoline, diesel, and coal are combusted, the same basic reaction occurs, but it is not as clean as methane. There is incomplete combustion that produces various forms of smoke and ash and there are complex side reactions that form other pollutants. Also those fuels are not generally 100% pure (especially coal) so other pollutants like mercury are also released.
But the same basic combustion reaction happens. Oxygen reacts with the hydrocarbon to produce carbon dioxide and water and releases lots of energy.
You can use chemistry and a technique called stoichiometry to calculate precisely how many kg of CO2 is released when a kg of some hydrocarbon like gasoline is combusted. That is the sort of thing we torture HS chemistry students with. For methane you get about 3.5 kg of CO2 for every kg of a petrochemical that you burn. The increase in mass is because you are replacing the light hydrogen atoms with heavier oxygen atoms from the atmosphere when you turn something like methane into carbon dioxide. For heavier petrochemicals like jet fuel, coal, and diesel the ratio is even higher.
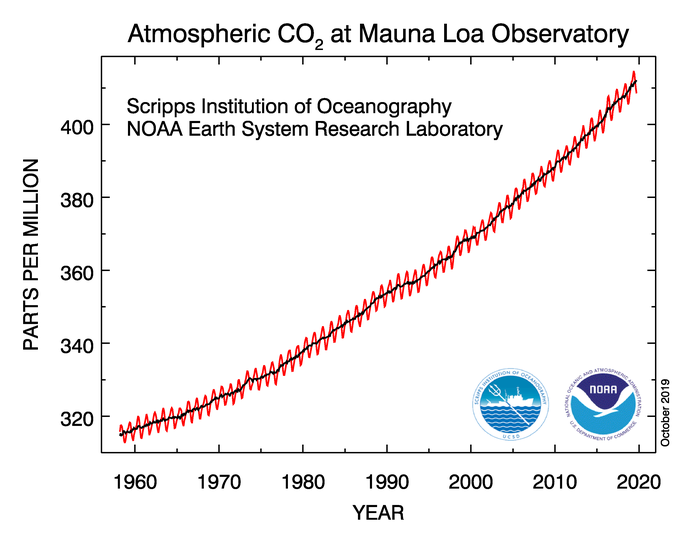
Josh is wrong, however, about CO2 in the atmosphere being stable. It has been increasing steadily since the industrial revolution. And since CO2 has a half life of about 100 years, our atmosphere still has lingering CO2 from coal and diesel that we burned 100 years ago.
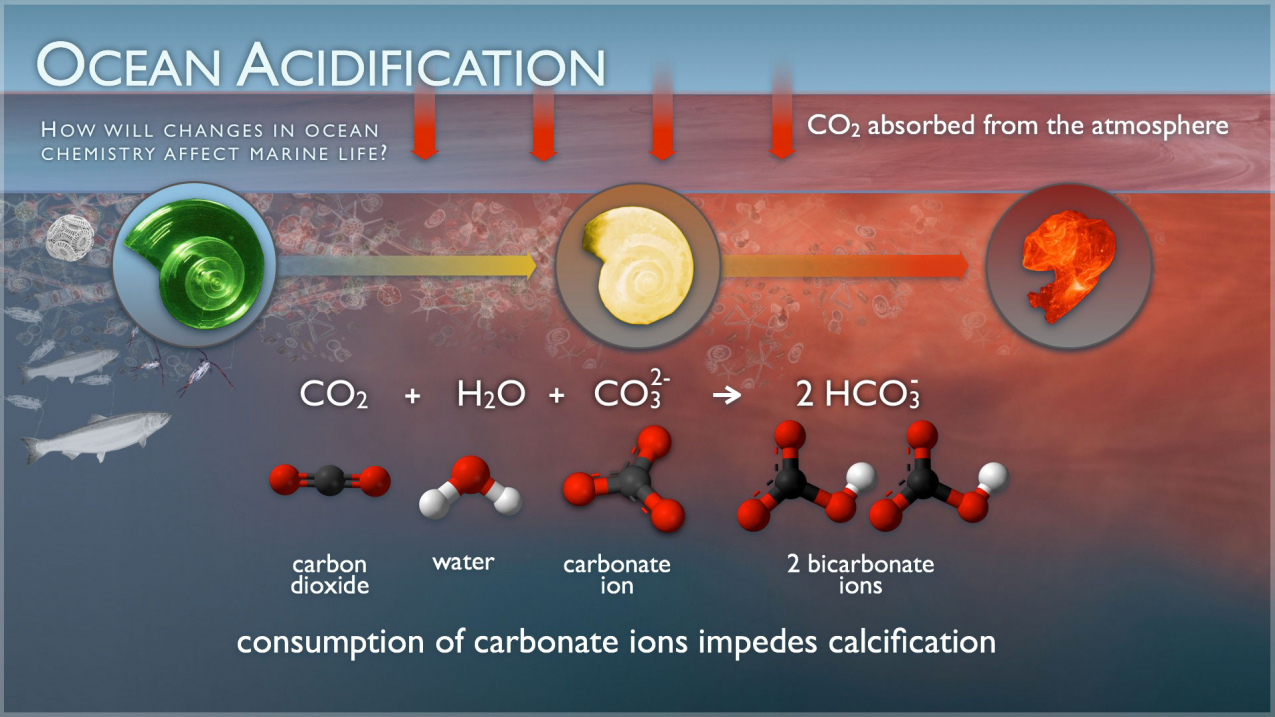
And yes, the oceans absorb about 25% of the man-made CO2 that is emitted into the atmosphere. Which is good and bad. Good in the CO2 in the oceans is not adding to the atmospheric greenhouse effect. But bad in that CO2 reacts with seawater and carbonate ions to form carbonic acid. which is making the oceans more acidic. And that has potentially dire consequences for the vast numbers of marine species that form calcium carbonate shells of any kind. So coral reefs, clams, and oysters to marine plankton at the base of the food chain like pteropods are all negatively affected by the increased acidity of the oceans.
In a chemical reaction, matter is neither created nor destroyed, it just changes form.
The increase in mass comes because in the combustion reaction, lighter hydrogen atoms are being stripped away from the jet fuel and are being replaced by heavier oxygen atoms. Which are coming from the atmosphere.
Petrochemicals like methane, gasoline, diesel, kerosene, coal, oil, etc. are hydrocarbon chains which means they are strands of carbon with hydrogen attached. The longer or bigger the hydrocarbon chain the more dense the liquid. Methane with 1 carbon is a gas, gasoline with 8 carbons is a liquid, motor oil and heavy greases have more carbons still, and bituminous coal, a solid, has 137 carbons.

When any hydrocarbon combusts it reacts with oxygen to form carbon dioxide and water. The simple reaction of methane is clean and just produces those two products and releases energy in the form of heat and light.

When larger hydrocarbons like gasoline, diesel, and coal are combusted, the same basic reaction occurs, but it is not as clean as methane. There is incomplete combustion that produces various forms of smoke and ash and there are complex side reactions that form other pollutants. Also those fuels are not generally 100% pure (especially coal) so other pollutants like mercury are also released.
But the same basic combustion reaction happens. Oxygen reacts with the hydrocarbon to produce carbon dioxide and water and releases lots of energy.
You can use chemistry and a technique called stoichiometry to calculate precisely how many kg of CO2 is released when a kg of some hydrocarbon like gasoline is combusted. That is the sort of thing we torture HS chemistry students with. For methane you get about 3.5 kg of CO2 for every kg of a petrochemical that you burn. The increase in mass is because you are replacing the light hydrogen atoms with heavier oxygen atoms from the atmosphere when you turn something like methane into carbon dioxide. For heavier petrochemicals like jet fuel, coal, and diesel the ratio is even higher.
Josh is wrong, however, about CO2 in the atmosphere being stable. It has been increasing steadily since the industrial revolution. And since CO2 has a half life of about 100 years, our atmosphere still has lingering CO2 from coal and diesel that we burned 100 years ago.

And yes, the oceans absorb about 25% of the man-made CO2 that is emitted into the atmosphere. Which is good and bad. Good in the CO2 in the oceans is not adding to the atmospheric greenhouse effect. But bad in that CO2 reacts with seawater and carbonate ions to form carbonic acid. which is making the oceans more acidic. And that has potentially dire consequences for the vast numbers of marine species that form calcium carbonate shells of any kind. So coral reefs, clams, and oysters to marine plankton at the base of the food chain like pteropods are all negatively affected by the increased acidity of the oceans.

0 x
A fool can throw out more questions than a wise man can answer. -RZehr